The biotech real estate market is experiencing unprecedented growth in 2026, with specialized laboratory facilities commanding premium valuations across major research hubs. Yet behind every successful life sciences facility lies a critical foundation that many overlook: precision surveying. Unlike conventional commercial properties, surveying for life sciences facilities: precision requirements for lab and cleanroom site assessments demands extraordinary accuracy, specialized equipment, and deep understanding of vibration-sensitive environments and temperature-controlled spaces. A single millimeter of deviation can compromise million-dollar research equipment or invalidate years of clinical trials.
As pharmaceutical companies, biotech startups, and research institutions compete for limited specialized space, the demand for facilities housing vibration-sensitive electron microscopes, ultra-cold storage units, and ISO-certified cleanrooms has skyrocketed. These environments require surveying tolerances that exceed traditional construction standards by orders of magnitude—where conventional surveys might accept variations of several millimeters, life sciences facilities often demand sub-millimeter precision across entire floor plates.
Key Takeaways
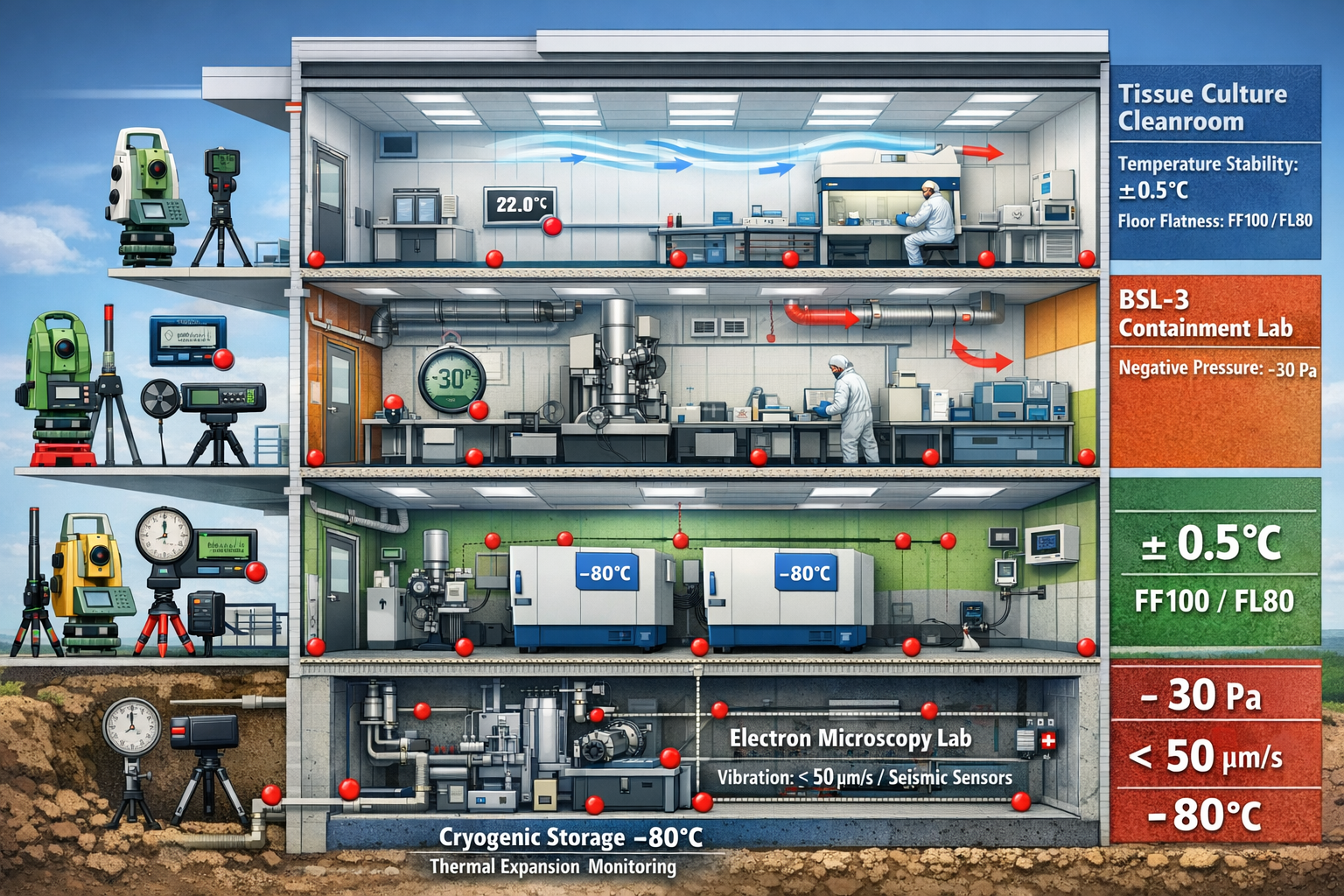
- Life sciences facilities require surveying precision 10-100 times greater than conventional commercial buildings, with floor flatness tolerances often specified at FF100/FL80 or higher for cleanroom environments
- Vibration-sensitive laboratories demand specialized site assessments measuring ambient vibration levels below 50 micrometers per second (μm/s) to protect electron microscopes and other precision instruments
- Cold storage and cryogenic facilities require thermal expansion assessments and continuous monitoring systems, as temperature variations of just 0.5°C can affect structural dimensions
- Cleanroom certification depends on precise dimensional control, with ISO Class 5 cleanrooms requiring floor levelness within ±0.5mm over 3 meters
- Specialized surveying equipment including laser interferometers, vibration analyzers, and 3D laser scanners are essential for meeting life sciences facility requirements
Understanding the Unique Demands of Life Sciences Facility Surveying

Why Life Sciences Facilities Require Extraordinary Precision
Life sciences facilities operate at the intersection of cutting-edge research, regulatory compliance, and extreme environmental control. Unlike standard commercial or industrial buildings, these specialized spaces house equipment and processes that are extraordinarily sensitive to physical conditions. 🔬
The precision requirements stem from several critical factors:
Equipment Sensitivity: Modern research instruments like transmission electron microscopes (TEMs), atomic force microscopes (AFMs), and mass spectrometers can detect vibrations measured in nanometers. These instruments often cost $500,000 to $5 million each and require installation on perfectly level, vibration-isolated foundations. Even minor floor irregularities can render these instruments unusable.
Process Criticality: Pharmaceutical manufacturing under Good Manufacturing Practice (GMP) regulations requires documented environmental stability. Temperature fluctuations, structural movement, or airflow disruptions can invalidate entire production batches worth millions of dollars.
Regulatory Compliance: Facilities must meet stringent standards from organizations including the FDA, EMA, and ISO. Documentation of dimensional accuracy and environmental conditions forms part of the regulatory submission package for drug approvals.
Cleanroom Classifications: ISO 14644 cleanroom standards specify particle counts per cubic meter, but achieving these classifications requires precise architectural control. Wall verticality, ceiling flatness, and floor levelness all impact airflow patterns that determine particle settlement.
Similar to how structural engineering services require detailed assessment of building integrity, life sciences surveying demands comprehensive documentation of every dimensional aspect of the facility.
The Booming Biotech Real Estate Market in 2026
The life sciences real estate sector has emerged as one of the fastest-growing commercial property segments in 2026. Major metropolitan areas including Boston, San Francisco, San Diego, and emerging hubs like Raleigh-Durham have seen vacancy rates for lab space drop below 2%, creating intense competition for suitable facilities.
Several factors drive this unprecedented demand:
| Market Driver | Impact on Surveying Requirements |
|---|---|
| mRNA Vaccine Production | Requires ultra-cold storage (-80°C) with thermal expansion monitoring |
| Cell and Gene Therapy | Demands BSL-2/BSL-3 containment with precise pressure differentials |
| Personalized Medicine | Needs flexible cleanroom configurations with modular precision |
| AI-Driven Drug Discovery | Requires vibration-free environments for robotic automation |
| Biotech Startups | Seek smaller, highly specialized spaces with exacting specifications |
This market pressure has created a premium on existing certified facilities and driven new construction of purpose-built lab buildings. Developers investing $500-$1,000 per square foot in life sciences construction cannot afford surveying errors that compromise certification or equipment installation.
The surveying profession has responded with specialized practitioners who understand both traditional land surveying principles and the unique requirements of controlled environments. Much like choosing between different types of property surveys, selecting the appropriate surveying approach for life sciences facilities requires matching methodology to specific facility requirements.
Critical Precision Requirements for Lab and Cleanroom Site Assessments
Floor Flatness and Levelness Specifications
Floor flatness (FF) and floor levelness (FL) represent the most critical dimensional requirements for life sciences facilities. These measurements, defined by ASTM E1155 (Face Floor Profile Numbers), quantify surface regularity at scales invisible to the naked eye.
For life sciences applications, typical specifications include:
- Standard Laboratory Space: FF50/FL40 minimum
- Cleanroom Environments: FF80/FL60 to FF100/FL80
- Precision Equipment Areas: FF100/FL100 or higher
- Vibration-Sensitive Labs: Custom specifications with localized tolerances of ±0.25mm over 3 meters
FF numbers measure the maximum floor curvature or waviness over a 24-inch (610mm) distance. Higher numbers indicate flatter floors. FL numbers measure overall levelness or tilt across the entire floor. These measurements are captured using specialized equipment including:
- Laser-based floor profilers that record elevation data at 12-inch intervals
- Digital levels with 0.01mm resolution for critical areas
- Total stations with automatic target recognition for large-area mapping
- 3D laser scanners generating millions of data points for comprehensive analysis
The surveying process for floor assessment typically involves:
- Grid establishment at 5-10 foot intervals across the entire floor plate
- Elevation measurement at each grid intersection point
- Statistical analysis calculating FF/FL numbers according to ASTM standards
- Deviation mapping showing areas requiring remediation
- Certification documentation for regulatory submissions
Achieving these tolerances requires coordination between surveyors, concrete contractors, and equipment installers. The surveyor's role extends beyond measurement to include pre-pour planning, real-time monitoring during concrete placement, and post-cure verification.
Just as building surveys provide comprehensive property assessments, floor flatness surveys deliver the dimensional foundation for life sciences facility certification.
Vibration Assessment and Mitigation Requirements
Vibration control represents perhaps the most challenging aspect of surveying for life sciences facilities: precision requirements for lab and cleanroom site assessments. Research-grade instruments can detect vibrations measured in micrometers (μm) or even nanometers (nm), making ambient vibration assessment critical before site selection or equipment installation.
Vibration Criteria for Life Sciences Equipment
Different instruments require different vibration environments, typically specified using VC curves (Vibration Criterion curves) developed by BBN Technologies:
- VC-A (50 μm/s): Suitable for optical microscopes and basic lab equipment
- VC-B (25 μm/s): Required for high-power optical microscopes and microbalances
- VC-C (12.5 μm/s): Necessary for scanning electron microscopes (SEMs)
- VC-D (6 μm/s): Essential for transmission electron microscopes (TEMs)
- VC-E (3 μm/s): Critical for atomic force microscopes and STMs
- VC-F (1.5 μm/s): Required for electron beam lithography and ultra-precision manufacturing
Site Vibration Assessment Methodology
Conducting a proper vibration survey involves:
1. Source Identification: Mapping potential vibration sources including:
- Adjacent roadways and traffic patterns
- Nearby rail lines (surface and subway)
- Building mechanical systems (HVAC, pumps, elevators)
- Manufacturing equipment in adjacent spaces
- Construction activities in surrounding areas
2. Ambient Measurement: Installing seismometers or accelerometers to record vibration levels over 24-72 hours, capturing:
- Peak vibration velocities in three axes (X, Y, Z)
- Frequency spectrum analysis identifying dominant frequencies
- Time-domain analysis correlating vibrations with specific events
- Statistical analysis of vibration occurrence and duration
3. Predictive Modeling: Using finite element analysis (FEA) to predict:
- Vibration transmission through building structure
- Effectiveness of proposed isolation systems
- Impact of new equipment installations on existing instruments
4. Mitigation Design: Specifying appropriate isolation solutions:
- Passive isolation: Spring-based or pneumatic tables (effective above 5-10 Hz)
- Active isolation: Electronic feedback systems (effective below 5 Hz)
- Structural isolation: Building-level solutions including separate foundations
- Operational controls: Restricting activities during sensitive measurements
The surveyor must work closely with vibration specialists and structural engineers to translate measurement data into actionable design requirements. This interdisciplinary approach mirrors the comprehensive nature of structural surveys that assess multiple building systems simultaneously.
Temperature Stability and Thermal Expansion Considerations
Life sciences facilities often contain extreme temperature environments ranging from cryogenic storage at -196°C (liquid nitrogen) to incubation chambers at +37°C. These temperature variations create thermal expansion and contraction that must be anticipated and accommodated in facility design.
Thermal Expansion Calculations for Survey Planning
Different materials expand at different rates, quantified by their coefficient of thermal expansion (CTE):
- Concrete: 10-14 × 10⁻⁶ per °C
- Steel: 11-13 × 10⁻⁶ per °C
- Aluminum: 22-24 × 10⁻⁶ per °C
- Stainless Steel: 16-18 × 10⁻⁶ per °C
For a 30-meter concrete floor experiencing a 10°C temperature change, the dimensional change would be:
ΔL = L × CTE × ΔT = 30,000mm × 12 × 10⁻⁶ × 10°C = 3.6mm
This 3.6mm expansion can compromise equipment alignment, door operation, and cleanroom seal integrity. Surveyors must:
- Establish reference temperatures for all dimensional measurements
- Document environmental conditions during survey work
- Calculate expected dimensional changes across operating temperature ranges
- Specify expansion joints and flexible connections at appropriate intervals
- Plan for seasonal variations in uncontrolled spaces
Cold Storage and Cryogenic Facility Surveying
Ultra-cold storage facilities present unique surveying challenges:
Walk-in Freezers (-20°C to -40°C):
- Floor insulation creates elevation differences requiring ramp transitions
- Thermal bridging at penetrations must be documented
- Door clearances must account for frost buildup and gasket compression
Ultra-Low Temperature Freezers (-80°C):
- Equipment generates significant heat requiring HVAC capacity
- Floor loading from multiple units (800-1,200 lbs each) requires structural verification
- Vibration from compressors may affect adjacent sensitive equipment
Cryogenic Storage (-196°C liquid nitrogen):
- Specialized flooring systems resist thermal shock
- Emergency ventilation systems require precise positioning
- Oxygen displacement sensors must be surveyed into exact locations per code
The surveying process for these facilities requires continuous monitoring rather than single-point-in-time measurements. Modern surveying increasingly employs permanent reference monuments with automated total stations that track dimensional changes over time, providing early warning of structural movement or settlement.
This approach parallels the ongoing monitoring provided by stock condition surveys that track building deterioration over extended periods.
Cleanroom Certification and Dimensional Control Standards
ISO 14644 Cleanroom Classification Requirements
ISO 14644 represents the international standard for cleanroom classification, specifying maximum allowable particle concentrations for different cleanliness levels. While primarily focused on air quality, achieving these classifications requires precise dimensional control of the physical environment.
Cleanroom Class Definitions
| ISO Class | Particles ≥0.5μm per m³ | Typical Applications |
|---|---|---|
| ISO 3 | 1,000 | Semiconductor manufacturing, advanced research |
| ISO 4 | 10,000 | Semiconductor, pharmaceutical aseptic processing |
| ISO 5 | 100,000 | Pharmaceutical filling operations, medical device assembly |
| ISO 6 | 1,000,000 | Pharmaceutical manufacturing, medical device packaging |
| ISO 7 | 10,000,000 | General pharmaceutical production, cosmetics |
| ISO 8 | 100,000,000 | Pharmaceutical secondary packaging, food processing |
Dimensional Requirements Supporting Cleanroom Performance
Achieving and maintaining these particle counts requires architectural precision:
Wall Verticality: Walls must be plumb within ±3mm over floor-to-ceiling height to:
- Ensure proper seal of wall-to-ceiling joints
- Prevent particle accumulation in corners
- Maintain uniform airflow patterns
- Support modular cleanroom panel systems
Ceiling Flatness: HEPA filter ceilings require levelness within ±6mm across entire ceiling plane to:
- Ensure uniform downward laminar airflow
- Prevent air velocity variations that affect particle settlement
- Maintain consistent filter-to-filter spacing
- Support proper gasket compression for leak-tight seals
Floor Tolerances: As previously discussed, FF/FL specifications directly impact:
- Cleanroom equipment leveling and stability
- Drainage patterns in wet cleanrooms
- Seamless flooring installation (epoxy, vinyl, terrazzo)
- Modular raised floor systems for underfloor HVAC
Survey Documentation for Cleanroom Certification
Cleanroom certification requires comprehensive documentation including:
- As-built dimensional drawings showing actual constructed conditions
- Deviation reports documenting variances from design specifications
- Photographic documentation of critical junctions and penetrations
- Material certifications for flooring, wall panels, and ceiling systems
- Airflow visualization studies correlating physical dimensions to performance
Professional surveyors provide this documentation package, which becomes part of the facility's Master Validation Plan required for FDA or EMA regulatory approval. The surveyor's certification that dimensional tolerances meet specifications provides crucial third-party verification.
Pressure Differential Monitoring and Spatial Relationships
Beyond dimensional precision, life sciences facilities require careful surveying of spatial relationships between different classified areas. Pressure cascades—progressive pressure differentials between adjacent spaces—prevent contamination migration and maintain containment.
Pressure Cascade Design Requirements
Typical pressure differentials include:
- ISO 5 to ISO 6: +5 to +15 Pascals (Pa)
- ISO 6 to ISO 7: +5 to +15 Pa
- ISO 7 to ISO 8: +5 to +15 Pa
- Controlled to Uncontrolled: +10 to +20 Pa
- BSL-3 Containment: -30 to -50 Pa (negative pressure)
Maintaining these differentials requires:
Precise Door Positioning: Doors between classified areas must:
- Seal completely with gaskets compressed uniformly (requires frame squareness within ±1mm)
- Include vision panels positioned for workflow observation
- Incorporate interlocks preventing simultaneous opening
- Maintain specified swing clearances despite floor coatings
HVAC Duct Penetration Mapping: Supply and return air penetrations must be surveyed to verify:
- Correct positioning relative to process equipment
- Adequate clearance for maintenance access
- Proper sealing preventing bypass airflow
- Compliance with minimum distance requirements from critical processes
Pass-Through and Airlock Dimensions: Material transfer systems require:
- Precise alignment of doors on opposite sides
- Adequate interior dimensions for material carts or containers
- Proper elevation matching adjacent floor levels
- Correct positioning of UV sterilization lamps or HEPA filters
The surveyor's role includes verifying these spatial relationships during construction and documenting compliance. This verification process shares similarities with specific defect reports that identify and document particular building issues requiring remediation.
Advanced Surveying Technologies for Life Sciences Applications
3D Laser Scanning and Building Information Modeling (BIM)
The complexity of surveying for life sciences facilities: precision requirements for lab and cleanroom site assessments has driven adoption of advanced surveying technologies that capture comprehensive dimensional data far beyond traditional methods.
3D Laser Scanning Capabilities
Modern terrestrial laser scanners capture millions of measurement points per second, creating detailed "point clouds" representing every surface in the scanned environment. For life sciences facilities, this technology offers:
Comprehensive Documentation:
- Complete as-built records of existing facilities before renovation
- Verification of new construction against design models
- Detection of conflicts between architectural, structural, and MEP systems
- Documentation of complex equipment installations
Precision Measurement:
- Accuracy of ±1-3mm at ranges up to 100 meters
- Sub-millimeter precision in controlled environments
- Measurement of inaccessible areas (high ceilings, confined spaces)
- Non-contact measurement preventing contamination in clean environments
Rapid Data Acquisition:
- Complete facility scan in hours versus days for traditional methods
- Minimal disruption to ongoing operations
- Reduced time in contamination-controlled areas
- Immediate quality verification during construction
BIM Integration for Life Sciences Projects
Building Information Modeling (BIM) has become standard practice for life sciences facility design and construction. Surveying data integrates with BIM workflows through:
Scan-to-BIM Processes:
- Laser scan existing facility creating point cloud
- Register point cloud to project coordinate system
- Model architectural elements (walls, floors, ceilings) from point cloud
- Extract equipment locations and dimensions
- Compare as-built conditions to design intent
- Generate deviation reports highlighting discrepancies
Clash Detection:
- Identify conflicts between designed systems before construction
- Verify clearances for equipment installation and maintenance
- Ensure compliance with minimum spacing requirements
- Validate accessibility for cleaning and sterilization
Lifecycle Facility Management:
- Maintain accurate as-built BIM models for future renovations
- Track equipment locations and specifications
- Plan equipment upgrades within existing constraints
- Support regulatory inspections with comprehensive documentation
The integration of surveying and BIM creates a digital twin of the facility—a virtual representation maintaining dimensional accuracy throughout the building lifecycle. This approach proves particularly valuable for life sciences facilities undergoing frequent reconfiguration to accommodate evolving research needs.
Automated Monitoring Systems and Continuous Verification
Traditional surveying provides snapshots of conditions at specific moments. Life sciences facilities increasingly require continuous monitoring to detect dimensional changes, settlement, or structural movement that could compromise operations.
Permanent Survey Monuments and Automated Total Stations
Robotic total stations can be permanently installed in facilities, automatically measuring to reference targets on a scheduled basis:
Settlement Monitoring:
- Track vertical movement of floor slabs over time
- Detect differential settlement affecting equipment alignment
- Provide early warning of foundation issues
- Generate trend data for predictive maintenance
Structural Deformation Monitoring:
- Measure building deflection under loading
- Monitor thermal expansion and contraction cycles
- Verify structural performance during adjacent construction
- Document compliance with deformation limits
Equipment Position Verification:
- Confirm critical equipment remains within alignment tolerances
- Track movement of vibration-isolated platforms
- Verify stability of precision instruments
- Support recalibration scheduling
Environmental Monitoring Integration
Modern surveying systems integrate with building automation systems (BAS) to correlate dimensional changes with environmental conditions:
- Temperature sensors tracking thermal gradients across spaces
- Humidity monitors detecting conditions affecting materials
- Vibration sensors providing continuous vibration level data
- Pressure transducers verifying room pressure differentials
- Particle counters confirming cleanroom classification
This integrated approach creates a comprehensive understanding of facility performance, identifying relationships between environmental conditions and dimensional stability. The data supports both immediate operational decisions and long-term facility planning.
Similar to how property certification services verify building compliance, continuous monitoring systems provide ongoing verification of life sciences facility performance.
Regulatory Compliance and Documentation Requirements
FDA and GMP Facility Qualification Standards
Life sciences facilities producing pharmaceuticals, biologics, or medical devices must comply with Good Manufacturing Practice (GMP) regulations enforced by the FDA (United States), EMA (Europe), and other regulatory agencies worldwide. Surveying documentation forms a critical component of facility qualification.
The Qualification Lifecycle
Facility qualification follows a structured process:
1. User Requirements Specification (URS):
- Defines functional and performance requirements
- Specifies dimensional tolerances and environmental conditions
- Establishes acceptance criteria for qualification
- Documents regulatory requirements
2. Design Qualification (DQ):
- Verifies design meets URS requirements
- Reviews design drawings and specifications
- Confirms appropriate materials and construction methods
- Includes preliminary surveying feasibility assessment
3. Installation Qualification (IQ):
- Documents equipment and system installation
- Surveying verifies equipment positioning and alignment
- Confirms utilities meet specifications
- Photographs and measures critical installations
4. Operational Qualification (OQ):
- Tests systems across operating ranges
- Surveying confirms dimensional stability under operating conditions
- Verifies environmental control system performance
- Documents system capabilities
5. Performance Qualification (PQ):
- Demonstrates facility performs as intended for actual production
- Surveying monitors dimensional stability during production campaigns
- Confirms sustained compliance with specifications
- Establishes ongoing monitoring requirements
Surveying Documentation for Regulatory Submissions
Survey deliverables supporting regulatory compliance include:
As-Built Drawings:
- Dimensioned floor plans showing equipment locations
- Elevation views documenting vertical clearances
- Section drawings through critical areas
- Utility routing and connection points
Dimensional Verification Reports:
- Floor flatness and levelness test results
- Wall verticality measurements
- Ceiling levelness documentation
- Door and frame squareness verification
Equipment Installation Records:
- Photographs showing equipment positioning
- Alignment measurements for critical instruments
- Leveling documentation for process equipment
- Anchor bolt location verification
Environmental Monitoring Data:
- Temperature mapping results
- Pressure differential verification
- Vibration assessment reports
- Cleanroom certification test results
This documentation must be maintained throughout the facility lifecycle, with periodic reverification following any modifications. The surveyor's role extends beyond initial construction to include change control documentation for renovations and equipment installations.
International Standards and Best Practices
Beyond regulatory requirements, life sciences facility surveying follows industry standards and guidelines from professional organizations:
Key Standards and Guidelines
ISPE (International Society for Pharmaceutical Engineering):
- Baseline Guides for pharmaceutical facilities
- Good Practice Guides for specific facility types
- Risk-based approach to facility design and qualification
IEST (Institute of Environmental Sciences and Technology):
- Recommended practices for cleanroom design and testing
- Contamination control standards
- Environmental monitoring guidelines
ASHRAE (American Society of Heating, Refrigerating and Air-Conditioning Engineers):
- HVAC design standards for laboratories
- Ventilation requirements for health care facilities
- Energy efficiency guidelines
ASTM International:
- E1155: Standard Test Method for Determining FF and FL Numbers
- E2659: Standard Practice for Certificate of Calibration
- E2270: Standard Practice for Periodic Inspection of Building Facades
These standards provide the technical foundation for surveying specifications and acceptance criteria. Professional surveyors working in life sciences must maintain familiarity with applicable standards and participate in continuing education to stay current with evolving requirements.
The comprehensive nature of these requirements parallels the detailed assessment provided by commercial building surveys that evaluate all aspects of commercial property condition.
Site Selection and Pre-Construction Assessment Strategies
Evaluating Potential Sites for Life Sciences Development
Before committing to a site for life sciences facility development, comprehensive surveying and assessment identifies potential challenges that could compromise facility performance or inflate construction costs.
Critical Site Assessment Factors
Geotechnical Conditions:
- Soil bearing capacity for heavy equipment loads
- Groundwater levels affecting foundation design
- Soil contamination requiring remediation
- Expansive soils causing foundation movement
- Seismic considerations for vibration-sensitive equipment
Professional soil and water contamination assessments identify environmental issues that could delay or prevent development.
Ambient Vibration Environment:
- Proximity to highways, railways, and airports
- Adjacent industrial operations
- Underground utilities (water mains, sewers)
- Seismic activity and microseismic background
- Future development plans for surrounding areas
A baseline vibration survey conducted over 72 hours captures typical conditions, identifying:
- Peak vibration events and their sources
- Dominant vibration frequencies
- Diurnal patterns (day/night variations)
- Seasonal variations (traffic, construction)
Utility Infrastructure:
- Electrical service capacity for high-demand lab equipment
- Water quality and pressure for process and emergency systems
- Wastewater treatment capacity for chemical/biological effluent
- Natural gas availability for backup generators and lab equipment
- Telecommunications infrastructure for data-intensive research
Zoning and Regulatory Considerations:
- Permitted uses for research and manufacturing
- Height and setback restrictions
- Environmental permits for emissions and waste
- Biosafety containment requirements
- Community acceptance of biotech facilities
Adaptive Reuse Versus New Construction
The tight life sciences real estate market has driven interest in converting existing buildings to laboratory use. Surveying plays a crucial role in feasibility assessment:
Structural Capacity Evaluation:
- Floor loading capacity (labs require 100-150 psf versus 50-80 psf for offices)
- Floor-to-floor heights (labs need 14-18 feet versus 9-12 feet for offices)
- Column spacing accommodating lab modules
- Roof capacity for HVAC equipment (labs require 3-5x office HVAC capacity)
Dimensional Assessment:
- Floor flatness and levelness of existing slabs
- Structural bay sizes matching modular lab layouts
- Ceiling heights accommodating ductwork and utilities
- Core locations supporting cleanroom layouts
Renovation Feasibility:
- Cost to achieve required floor flatness
- Structural reinforcement requirements
- MEP infrastructure upgrade costs
- Schedule impact of working in occupied buildings
A comprehensive pre-acquisition survey comparing renovation costs to new construction often reveals that only specific building types—typically newer office buildings or previous industrial facilities—can be economically converted to life sciences use.
Specialized Equipment and Instrumentation for Life Sciences Surveying
Precision Measurement Tools and Calibration Requirements
Achieving the extraordinary precision required for surveying for life sciences facilities: precision requirements for lab and cleanroom site assessments demands specialized equipment maintained to the highest calibration standards.
Essential Surveying Instruments
Total Stations with Enhanced Accuracy:
- Angular accuracy: ±0.5 arc seconds or better
- Distance accuracy: ±(0.6mm + 1ppm) or better
- Automatic target recognition (ATR) for remote measurement
- Motorized tracking for dynamic measurements
- Environmental sensors correcting for temperature and pressure
Digital Levels:
- Resolution: 0.01mm for critical applications
- Accuracy: ±0.3mm per kilometer of leveling
- Electronic data recording eliminating transcription errors
- Automatic compensation for instrument tilt
- Bar-code staff reading for rapid measurements
Laser Interferometers:
- Measurement resolution: 0.001mm (1 micron)
- Accuracy: ±0.5 parts per million (ppm)
- Range: up to 80 meters
- Applications: precision equipment alignment, machine tool calibration
- Environmental compensation for temperature, humidity, pressure
3D Laser Scanners:
- Point accuracy: ±1mm at 10 meters
- Scan rate: 1,000,000+ points per second
- Range: 0.5 to 300+ meters depending on model
- HDR photography for photorealistic models
- Real-time registration and quality verification
Vibration Monitoring Equipment:
- Triaxial seismometers or accelerometers
- Frequency range: 0.1 Hz to 200 Hz
- Velocity sensitivity: 0.1 μm/s or better
- Data logging: continuous recording for 24-72 hours
- Spectrum analysis: identifying dominant frequencies
Calibration and Traceability
All surveying equipment used for life sciences facilities must maintain calibration traceability to national standards (NIST in the United States):
Calibration Frequency:
- Annual calibration minimum for all precision instruments
- Semi-annual or quarterly calibration for instruments used in critical applications
- Field verification before each project using certified check standards
- Immediate recalibration following any impact, drop, or suspected damage
Calibration Documentation:
- Certificate of calibration with measurement uncertainties
- Traceability chain to national standards
- Before and after calibration data
- Adjustment records if calibration required correction
- Next calibration due date
Environmental Controls:
- Temperature-controlled storage for instruments
- Humidity control preventing corrosion and drift
- Shock-isolated transport cases
- Regular cleaning and maintenance
- Firmware updates from manufacturers
The investment in precision equipment and rigorous calibration protocols ensures that measurements meet the demanding tolerances required for life sciences applications. This commitment to accuracy parallels the thoroughness of RICS specialist defect surveys that investigate specific building issues requiring expert assessment.
Case Studies: Surveying Challenges in Real-World Life Sciences Projects
Ultra-Cold Storage Facility: Thermal Management and Structural Monitoring
A major pharmaceutical company required a dedicated -80°C ultra-low temperature (ULT) storage facility for COVID-19 vaccine storage and distribution. The facility would house 200 ULT freezers in a 10,000 square foot space, presenting unique surveying challenges.
Project Requirements
Environmental Specifications:
- Ambient temperature: 18-22°C (±2°C)
- Relative humidity: 30-50%
- Floor flatness: FF80/FL60 minimum
- Vibration: VC-A (50 μm/s) maximum
Structural Considerations:
- Each ULT freezer: 400-800 lbs loaded weight
- Total floor loading: 160,000 lbs over 10,000 sf = 16 psf (within capacity)
- Heat rejection: 200 freezers × 1.5 kW = 300 kW total
- Backup power requirements for critical inventory protection
Surveying Approach
Pre-Construction Assessment:
- Vibration survey identified nearby loading dock as potential issue
- Floor flatness verification of existing warehouse slab
- Structural capacity review confirming adequate floor strength
- Thermal modeling predicting temperature gradients
Construction Monitoring:
- Floor grinding and polishing to achieve FF80/FL60 specification
- Re-survey verification after floor preparation
- Installation layout marking exact freezer positions
- Leveling verification for each freezer installation
Post-Installation Monitoring:
- Permanent survey monuments established at building columns
- Quarterly monitoring detecting any floor settlement
- Thermal expansion tracking as freezers reached operating temperature
- Vibration verification confirming loading dock isolation effective
Outcomes and Lessons Learned
The facility successfully achieved certification and has operated for two years without dimensional issues. Key lessons included:
- Temperature stabilization period: Floor dimensions shifted 2-3mm during first month as thermal equilibrium established
- Vibration isolation: Retrofitting loading dock with vibration-damped doors reduced peak vibrations by 60%
- Monitoring value: Quarterly surveys detected minor settlement (1.5mm) allowing proactive freezer releveling
Vibration-Sensitive Electron Microscopy Laboratory: Site Selection and Foundation Design
A research university planned a new Materials Science Research Center housing multiple transmission electron microscopes (TEMs) requiring VC-D vibration criteria (6 μm/s maximum). Site selection and foundation design depended heavily on surveying and vibration assessment.
Site Evaluation Process
Three potential sites evaluated:
Site A – Urban Campus Core:
- Adjacent to subway line (50 meters distance)
- Vibration survey: 15-25 μm/s peak levels
- Conclusion: Unacceptable without extraordinary mitigation
Site B – Campus Perimeter:
- Near major highway (100 meters distance)
- Vibration survey: 8-12 μm/s peak levels
- Conclusion: Marginal, requiring significant isolation
Site C – Research Park:
- Isolated location, minimal traffic
- Vibration survey: 3-5 μm/s peak levels
- Conclusion: Acceptable with standard isolation
Site C selected despite higher land costs, as vibration mitigation costs at other sites exceeded land premium.
Foundation Design and Verification
Isolated Foundation System:
- Separate foundation for TEM laboratory
- 12-inch separation gap from main building structure
- Pneumatic isolation tables for individual instruments
- Mass-spring tuning to building natural frequencies
Surveying Verification:
- Foundation elevation survey confirming level within ±1mm
- Isolation gap verification ensuring complete structural separation
- Vibration testing at foundation level before equipment installation
- Equipment alignment survey for TEM installation
- Operational vibration verification with instruments running
Results
All TEMs achieved manufacturer specifications for imaging resolution, confirming vibration environment met requirements. Post-occupancy vibration monitoring showed:
- Ambient conditions: 2-4 μm/s (within VC-E criteria)
- Peak events: 5-7 μm/s during heavy rain (still within VC-D)
- Equipment operation: No measurable vibration transmission between instruments
The project demonstrated the critical importance of site selection based on surveying data rather than attempting to overcome poor sites through engineering solutions.
Future Trends in Life Sciences Facility Surveying

Emerging Technologies and Methodologies
The field of surveying for life sciences facilities: precision requirements for lab and cleanroom site assessments continues to evolve with advancing technology and changing industry needs.
Artificial Intelligence and Machine Learning
AI-enhanced surveying applications emerging in 2026 include:
Automated Point Cloud Analysis:
- Machine learning algorithms automatically identifying building elements
- Anomaly detection flagging dimensional deviations
- Predictive modeling forecasting settlement and movement
- Pattern recognition correlating environmental conditions with dimensional changes
Intelligent Monitoring Systems:
- AI analyzing continuous monitoring data
- Early warning of developing issues before tolerance exceedance
- Optimization of monitoring frequency based on risk assessment
- Integration with building automation for automated responses
Digital Twin Evolution:
- Real-time updating of BIM models from monitoring data
- Predictive maintenance scheduling based on dimensional trends
- Virtual reality facility walkthroughs for planning
- Augmented reality overlay of design intent on existing conditions
Miniaturization and Distributed Sensing
Wireless sensor networks enable unprecedented monitoring density:
MEMS-based Sensors:
- Micro-electromechanical systems (MEMS) accelerometers
- Wireless transmission eliminating installation costs
- Battery life measured in years
- Cost enabling hundreds of sensors per facility
Distributed Fiber Optic Sensing:
- Temperature measurement every meter along fiber
- Strain measurement detecting structural deformation
- Vibration detection at multiple points
- Single fiber monitoring entire facility
IoT Integration:
- Sensors communicating via building WiFi infrastructure
- Cloud-based data storage and analysis
- Mobile app access to real-time conditions
- Integration with facility management systems
Sustainability and Green Building Considerations
Life sciences facilities face increasing pressure to reduce environmental impact while maintaining rigorous performance standards. Surveying supports sustainability goals through:
Energy Optimization:
- Thermal imaging identifying heat loss
- Airflow visualization optimizing HVAC efficiency
- Daylighting analysis reducing artificial lighting loads
- Building envelope performance verification
Water Conservation:
- Leak detection through moisture mapping
- Condensation risk assessment
- Drainage system verification
- Rainwater harvesting system layout
Material Efficiency:
- Accurate as-built documentation enabling targeted renovations
- Waste reduction through precise material quantification
- Reuse facilitation through comprehensive facility documentation
- Lifecycle assessment supporting sustainable material selection
Green Building Certification:
- LEED documentation requirements
- WELL Building Standard verification
- Living Building Challenge compliance
- Energy modeling validation
The surveying profession increasingly serves as a bridge between sustainability objectives and operational requirements, demonstrating that environmental responsibility and research excellence need not conflict.
Conclusion: Ensuring Excellence Through Precision
The specialized field of surveying for life sciences facilities: precision requirements for lab and cleanroom site assessments represents the intersection of traditional surveying expertise and cutting-edge scientific research. As the biotech industry continues its rapid expansion in 2026, the demand for facilities that can support vibration-sensitive instruments, maintain ultra-cold storage environments, and provide certified cleanroom spaces has never been higher.
Success in this demanding field requires:
✅ Extraordinary precision – tolerances measured in fractions of millimeters across entire building floors
✅ Specialized equipment – laser interferometers, 3D scanners, and vibration analyzers maintained to the highest calibration standards
✅ Comprehensive documentation – detailed records supporting regulatory submissions and facility qualification
✅ Continuous monitoring – ongoing verification ensuring sustained compliance throughout facility lifecycle
✅ Interdisciplinary collaboration – working closely with architects, engineers, contractors, and facility operators
✅ Regulatory knowledge – understanding FDA, ISO, and industry standards governing life sciences facilities
Actionable Next Steps
For organizations planning life sciences facility projects:
1. Engage Specialized Surveyors Early: Include surveying expertise in initial site selection and feasibility assessment, not just construction verification.
2. Establish Clear Tolerance Requirements: Define specific FF/FL numbers, vibration criteria, and environmental specifications in project requirements.
3. Plan for Continuous Monitoring: Budget for permanent monitoring systems providing ongoing verification rather than point-in-time surveys.
4. Document Comprehensively: Maintain detailed as-built records supporting regulatory compliance and future modifications.
5. Invest in Quality: Recognize that precision surveying represents a small fraction of total project costs but prevents expensive failures.
For surveying professionals seeking to enter this specialized field:
1. Pursue Specialized Training: Develop expertise in floor flatness measurement, vibration assessment, and cleanroom standards.
2. Invest in Advanced Equipment: Acquire precision instruments and maintain rigorous calibration protocols.
3. Build Industry Knowledge: Study pharmaceutical manufacturing, biotech research, and regulatory requirements.
4. Develop Partnerships: Collaborate with architects, engineers, and contractors specializing in life sciences facilities.
5. Maintain Continuing Education: Stay current with evolving standards, technologies, and industry best practices.
The future of life sciences research—from personalized medicine to climate change solutions—depends on facilities that provide the precisely controlled environments enabling scientific breakthroughs. Professional surveying ensures these critical facilities meet the exacting standards required for success. 🔬
Whether planning a new facility, renovating existing space, or maintaining operating laboratories, the expertise provided through specialized life sciences surveying delivers the foundation for research excellence and regulatory compliance. The investment in precision measurement and comprehensive documentation pays dividends throughout the facility lifecycle, supporting the groundbreaking work that improves human health and advances scientific knowledge.
For comprehensive property assessment services across various building types, explore our range of professional surveying solutions tailored to your specific needs.
References
[1] The Joint Commission Physical Environment Survey Process 2025 And Beyond – https://eheinc.com/insights/blog/the-joint-commission-physical-environment-survey-process-2025-and-beyond/
[2] Business Occupancies In 2026 Jc Surveys – https://www.soleran.com/blog-posts/business-occupancies-in-2026-jc-surveys
[3] Fiscal Year 2026 Mission Priorities Document Mpd – https://www.cms.gov/files/document/fiscal-year-2026-mission-priorities-document-mpd.pdf
[8] 2026 Alta Nsps Land Title Survey Standards – https://www.partneresi.com/resources/references/standards-regulations/2026-alta-nsps-land-title-survey-standards/
[9] Criteria For Accrediting Applied And Natural Science Programs 2025 2026 – https://www.abet.org/accreditation/accreditation-criteria/criteria-for-accrediting-applied-and-natural-science-programs-2025-2026/





